Chicken wire (chemistry)


In chemistry, the term chicken wire is used in different contexts. Most of them relate to the similarity of the regular hexagonal (honeycomb-like) patterns found in certain chemical compounds to the mesh structure commonly seen in real chicken wire.
Examples
[edit]Polycyclic aromatic hydrocarbons
[edit]Polycyclic aromatic hydrocarbons or graphenes—including fullerenes, carbon nanotubes, and graphite—have a hexagonal structure that is often described as chicken wire-like.[1][2][3]

Hexagonal molecular structures
[edit]A hexagonal structure that is often described as chicken wire-like can also be found in other types of chemical compounds such as:
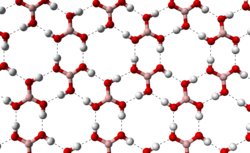
- Non-aromatic polycyclic hydrocarbons, e.g. steroids like cholesterol[4]
- Flat hexagonal hydrogen bonded trimesic acid (benzene-1,3,5-tricarboxylic acid),[5] boric acid, or melamine-cyanuric acid complexes[6]
- Interwoven molecule chains in the inorganic polymer NaAuS[7]
- Complexes of the protein clathrin[8]

Additional information
[edit]Bond line notation
[edit]The skeletal formula is a method to draw structural formulas of organic compounds where lines represent the chemical bonds and the vertices represent implicit carbon atoms.[9] This notation is sometimes called chicken wire notation by a Stanford professor.[10][11][12]

Chemical joke
[edit]It is an old joke[dubious – discuss] in chemistry to draw a polycyclic hexagonal chemical structure and call this fictional compound chickenwire.[citation needed] By adding one or two simple chemical groups to this skeleton, the compound can then be named following the official chemical naming convention. An example is 1,2-Dimethyl-chickenwire in a cartoon by Nick D. Kim.

Surface plots
[edit]In computational chemistry a chicken wire model or chicken wire surface plot is a way to visualize molecular models by drawing the polygon mesh of their surface (defined e.g. as the van der Waals radius or a certain electron density).[citation needed]
References
[edit]- ^ "Soccerballs". Calpoly.edu. Retrieved 2013-11-24.
- ^ "General Chemistry Online: Glossary". Antoine.frostburg.edu. Retrieved 2013-11-24.
- ^ "Space Chemicals: Scientific American". Sciam.com. Retrieved 2013-11-24.
- ^ "Get Healthy...Get Smart". Gethealthygetsmart.com. Archived from the original on 2012-07-17. Retrieved 2013-11-24.
- ^ Suzuki, Yuto; Histaki, Ichiro (12 October 2023). "Structural details of carboxylic acid-based Hydrogen-bonded Organic Frameworks (HOFs)". Polymer Journal. 56. Retrieved December 26, 2023.
- ^ Andrew D. Burrows (2004). "Crystal Engineering Using Multiple Hydrogen Bonds". Structure and Bonding. 108: 55–96. doi:10.1007/b14137. ISBN 978-3-540-20084-0. ISSN 0081-5993.
- ^ Axtell Ea, 3rd; Liao, JH; Kanatzidis, MG (October 1998). "Flux Synthesis of LiAuS and NaAuS: "Chicken-Wire-Like" Layer Formation by Interweaving of (AuS)(n)(n)(-) Threads. Comparison with alpha-HgS and AAuS (A = K, Rb)". Inorg Chem. 37 (21): 5583–5587. doi:10.1021/ic980360b. PMID 11670705.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ http://bio.winona.msus.edu/wilson/cell%20biology/unit3revANSWER.doc[permanent dead link]
- ^ Template Archived November 30, 2004, at the Wayback Machine
- ^ "Chem 32 Virtual Manual". Kalee.tock.com. Retrieved 2013-11-24.
- ^ "Stereochemistry and Chirality Part I Problems". Kalee.tock.com. 1995-11-07. Retrieved 2013-11-24.
- ^ "Chem 32 Virtual Manual". Kalee.tock.com. Retrieved 2013-11-24.
